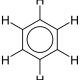
Hydrocarbons
Hydrocarbons include various organic compounds which are mainly composed of carbon and hydrogen. The C-H bonds in these molecules are covalent bonds. Light or heavy hydrocarbon is simply a trade name for classifying oil and petroleum products based on their distillation range and density. Each of these classifications can be subdivided based on the process. For example, light hydrocarbons can directly be produced through the distillation of crude oil and condensate, sweetening, and blending processes. In crude oil distillation, these hydrocarbons are typically obtained from the upper sections of the atmospheric distillation tower.
Light hydrocarbons typically fall within the distillation range of 40 to 390 ͦ C and have a density of 660 to 890.
Physical properties
Most hydrocarbons are nonpolar molecules. So, generally, they do not dissolve in polar solvents such as water, however, they dissolve in nonpolar solvents like oil (itself is a type of hydrocarbon). For instance, grease, which is a hydrocarbon from the alkane family, does not wash off hands with water because it is nonpolar. Therefore, nonpolar solvents like oil or gasoline are used to clean it.
Chemical properties
There is a significant difference between the chemical properties of aliphatic and aromatic hydrocarbons. Among aliphatic, the chemical behaviors of saturated and unsaturated hydrocarbons are different and unique.
All hydrocarbons are involved in a common combustion reaction. In this reaction, hydrocarbons rapidly react with oxygen, producing a significant amount of energy in the form of both light and heat (creating a flame), while also generating combustion products (such as water and carbon oxides).